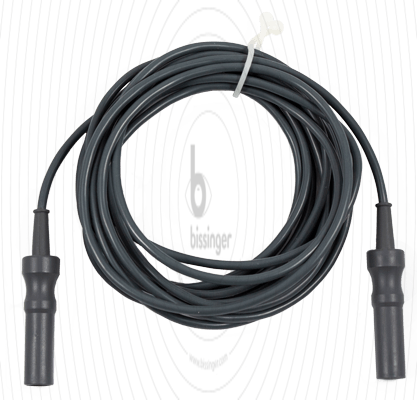
|
80100014 |
4 mm Socket |
Erbe T-Serie, Martin, Berchtold |
3 m |
|
|
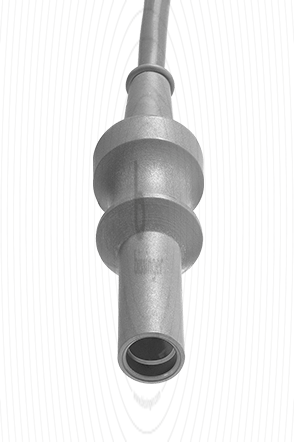
|
Cable connector instrument side
|
4 mm Socket
|
|
Cable plug generator side
|
Erbe T-Serie, Martin, Berchtold
|
|
Cable length
|
3 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418700146
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100015 |
4 mm Socket with Protection Sleeve |
Erbe T-Serie, Martin, Berchtold |
4 m |
|
|
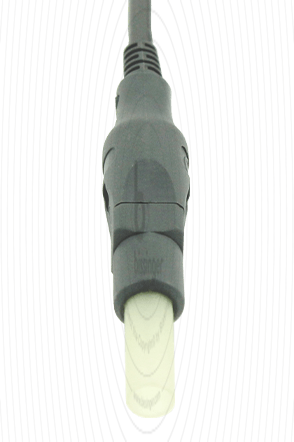
|
Cable connector instrument side
|
4 mm Socket with Protection Sleeve
|
|
Cable plug generator side
|
Erbe T-Serie, Martin, Berchtold
|
|
Cable length
|
4 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418746366
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100016 |
4 mm Socket |
Erbe T-Serie, Martin, Berchtold |
5 m |
|
|

|
Cable connector instrument side
|
4 mm Socket
|
|
Cable plug generator side
|
Erbe T-Serie, Martin, Berchtold
|
|
Cable length
|
5 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418700153
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100031 |
4 mm Socket |
Martin, Berchtold |
3 m |
|
|

|
Cable connector instrument side
|
4 mm Socket
|
|
Cable plug generator side
|
Martin, Berchtold
|
|
Cable length
|
3 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418700290
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100114 |
4 mm Socket |
Erbe ACC/ICC |
3 m |
|
|

|
Cable connector instrument side
|
4 mm Socket
|
|
Cable plug generator side
|
Erbe ACC/ICC
|
|
Cable length
|
3 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418700818
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100115 |
4 mm Socket |
Erbe ACC/ICC |
5 m |
|
|

|
Cable connector instrument side
|
4 mm Socket
|
|
Cable plug generator side
|
Erbe ACC/ICC
|
|
Cable length
|
5 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418700825
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100212 |
4 mm Plug |
Martin, Berchtold |
3 m |
|
|
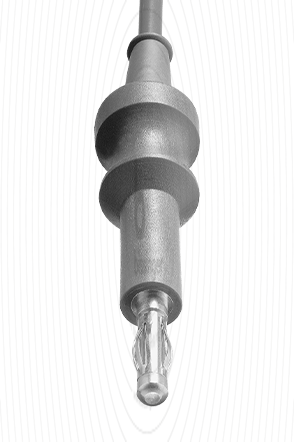
|
Cable connector instrument side
|
4 mm Plug
|
|
Cable plug generator side
|
Martin, Berchtold
|
|
Cable length
|
3 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701099
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100214 |
4 mm Plug |
Erbe T-Serie, Martin, Berchtold |
3 m |
|
|

|
Cable connector instrument side
|
4 mm Plug
|
|
Cable plug generator side
|
Erbe T-Serie, Martin, Berchtold
|
|
Cable length
|
3 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701105
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100215 |
4 mm Socket |
Bovie, Valleylab, Commed |
3 m |
|
|

|
Cable connector instrument side
|
4 mm Socket
|
|
Cable plug generator side
|
Bovie, Valleylab, Commed
|
|
Cable length
|
3 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701112
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100216 |
4 mm Socket |
Erbe T-Serie, Martin, Berchtold |
5 m |
|
|

|
Cable connector instrument side
|
4 mm Socket
|
|
Cable plug generator side
|
Erbe T-Serie, Martin, Berchtold
|
|
Cable length
|
5 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701129
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100217 |
4 mm Plug |
Bovie, Valleylab, Commed |
3 m |
|
|

|
Cable connector instrument side
|
4 mm Plug
|
|
Cable plug generator side
|
Bovie, Valleylab, Commed
|
|
Cable length
|
3 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701136
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100218 |
4 mm Plug |
Erbe Multifunctional Plug |
5 m |
|
|

|
Cable connector instrument side
|
4 mm Plug
|
|
Cable plug generator side
|
Erbe Multifunctional Plug
|
|
Cable length
|
5 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701143
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
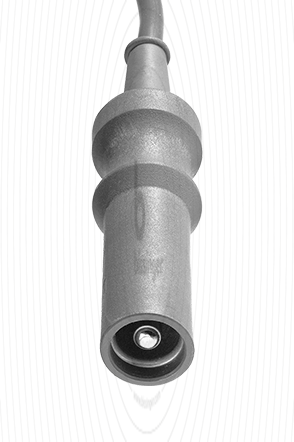
80100220 |
4 mm Plug protected |
Bovie, Valleylab, Commed |
3 m |
|
|
|
Cable connector instrument side
|
4 mm Plug protected
|
|
Cable plug generator side
|
Bovie, Valleylab, Commed
|
|
Cable length
|
3 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701167
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100221 |
4 mm Socket |
Bovie, Valleylab, Commed |
5 m |
|
|

|
Cable connector instrument side
|
4 mm Socket
|
|
Cable plug generator side
|
Bovie, Valleylab, Commed
|
|
Cable length
|
5 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701174
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100222 |
4 mm Plug protected |
Bovie, Valleylab, Commed |
5 m |
|
|

|
Cable connector instrument side
|
4 mm Plug protected
|
|
Cable plug generator side
|
Bovie, Valleylab, Commed
|
|
Cable length
|
5 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701181
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100223 |
4 mm Plug protected |
Erbe |
3 m |
|
|

|
Cable connector instrument side
|
4 mm Plug protected
|
|
Cable plug generator side
|
Erbe
|
|
Cable length
|
3 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418735308
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100230 |
4 mm Plug protected |
Martin, Berchtold |
3 m |
|
|

|
Cable connector instrument side
|
4 mm Plug protected
|
|
Cable plug generator side
|
Martin, Berchtold
|
|
Cable length
|
3 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701198
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
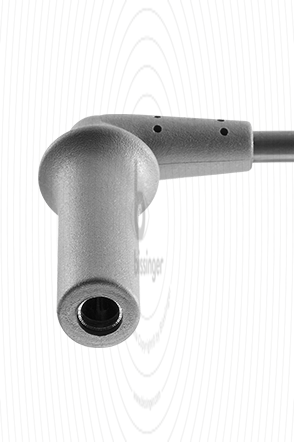
80100240 |
4 mm Socket angled |
Erbe T-Serie, Martin, Berchtold |
3 m |
|
|
|
Cable connector instrument side
|
4 mm Socket angled
|
|
Cable plug generator side
|
Erbe T-Serie, Martin, Berchtold
|
|
Cable length
|
3 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701204
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100241 |
4 mm Socket angled |
Erbe T-Serie, Martin, Berchtold |
5 m |
|
|

|
Cable connector instrument side
|
4 mm Socket angled
|
|
Cable plug generator side
|
Erbe T-Serie, Martin, Berchtold
|
|
Cable length
|
5 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418735094
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100242 |
4 mm Socket angled |
Bovie, Valleylab, Commed |
3 m |
|
|

|
Cable connector instrument side
|
4 mm Socket angled
|
|
Cable plug generator side
|
Bovie, Valleylab, Commed
|
|
Cable length
|
3 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701211
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100243 |
4 mm Socket angled |
Erbe ACC/ICC |
3 m |
|
|

|
Cable connector instrument side
|
4 mm Socket angled
|
|
Cable plug generator side
|
Erbe ACC/ICC
|
|
Cable length
|
3 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418734356
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100245 |
4 mm Socket angled |
Bovie, Valleylab, Commed |
5 m |
|
|

|
Cable connector instrument side
|
4 mm Socket angled
|
|
Cable plug generator side
|
Bovie, Valleylab, Commed
|
|
Cable length
|
5 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418732833
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100251 |
4 mm Socket with Protection Sleeve |
Bovie, Valleylab, Commed |
4 m |
|
|

|
Cable connector instrument side
|
4 mm Socket with Protection Sleeve
|
|
Cable plug generator side
|
Bovie, Valleylab, Commed
|
|
Cable length
|
4 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418746878
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
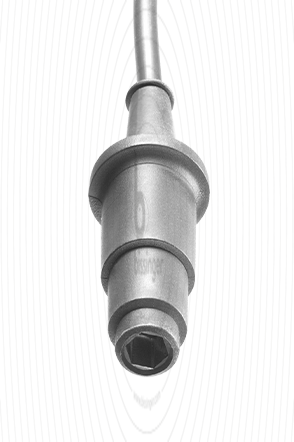
80100312 |
4 mm Hexagon Socket |
Bovie, Valleylab, Commed |
3 m |
|
|
|
Cable connector instrument side
|
4 mm Hexagon Socket
|
|
Cable plug generator side
|
Bovie, Valleylab, Commed
|
|
Cable length
|
3 m
|
|
Shaft diameter
|
0 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418742573
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
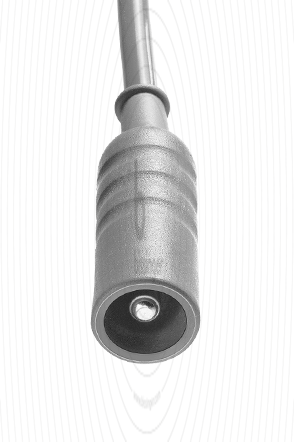
80100314 |
2 mm Plug protected for Resectoscope |
Erbe T-Serie, Martin, Berchtold |
3 m |
|
|
|
Cable connector instrument side
|
2 mm Plug protected for Resectoscope
|
|
Cable plug generator side
|
Erbe T-Serie, Martin, Berchtold
|
|
Cable length
|
3 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701266
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100315 |
4 mm Hexagon Socket |
Erbe T-Serie, Martin, Berchtold |
3 m |
|
|

|
Cable connector instrument side
|
4 mm Hexagon Socket
|
|
Cable plug generator side
|
Erbe T-Serie, Martin, Berchtold
|
|
Cable length
|
3 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701273
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100316 |
2 mm Plug protected for Resectoscope |
Martin, Berchtold |
3 m |
|
|

|
Cable connector instrument side
|
2 mm Plug protected for Resectoscope
|
|
Cable plug generator side
|
Martin, Berchtold
|
|
Cable length
|
3 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701280
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100317 |
3 mm Socket |
Erbe ACC/ICC |
5 m |
|
|
|
Cable connector instrument side
|
3 mm Socket
|
|
Cable plug generator side
|
Erbe ACC/ICC
|
|
Cable length
|
5 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701297
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100318 |
4 mm Hexagon Socket |
Erbe ACC/ICC |
3 m |
|
|

|
Cable connector instrument side
|
4 mm Hexagon Socket
|
|
Cable plug generator side
|
Erbe ACC/ICC
|
|
Cable length
|
3 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701303
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100319 |
3 mm Socket |
Erbe T-Serie, Martin, Berchtold |
3 m |
|
|

|
Cable connector instrument side
|
3 mm Socket
|
|
Cable plug generator side
|
Erbe T-Serie, Martin, Berchtold
|
|
Cable length
|
3 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701310
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100320 |
4 mm Socket angled |
Bovie, Valleylab, Commed |
3 m |
|
|

|
Cable connector instrument side
|
4 mm Socket angled
|
|
Cable plug generator side
|
Bovie, Valleylab, Commed
|
|
Cable length
|
3 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701327
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100322 |
3 mm Socket |
Bovie, Valleylab, Commed |
3 m |
|
|

|
Cable connector instrument side
|
3 mm Socket
|
|
Cable plug generator side
|
Bovie, Valleylab, Commed
|
|
Cable length
|
3 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418736695
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100324 |
2 mm Plug protected for Resectoscope |
Erbe T-Serie, Martin, Berchtold |
5 m |
|
|

|
Cable connector instrument side
|
2 mm Plug protected for Resectoscope
|
|
Cable plug generator side
|
Erbe T-Serie, Martin, Berchtold
|
|
Cable length
|
5 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701334
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100326 |
2 mm Plug protected for Resectoscope |
Martin, Berchtold |
5 m |
|
|

|
Cable connector instrument side
|
2 mm Plug protected for Resectoscope
|
|
Cable plug generator side
|
Martin, Berchtold
|
|
Cable length
|
5 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701341
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100330 |
2 mm Plug protected for Resectoscope |
Erbe ACC/ICC |
3 m |
|
|

|
Cable connector instrument side
|
2 mm Plug protected for Resectoscope
|
|
Cable plug generator side
|
Erbe ACC/ICC
|
|
Cable length
|
3 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701358
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100331 |
4 mm Socket |
Martin, Berchtold |
5 m |
|
|

|
Cable connector instrument side
|
4 mm Socket
|
|
Cable plug generator side
|
Martin, Berchtold
|
|
Cable length
|
5 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701365
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100340 |
2 mm Plug protected for Resectoscope |
Bovie, Valleylab, Commed |
5 m |
|
|

|
Cable connector instrument side
|
2 mm Plug protected for Resectoscope
|
|
Cable plug generator side
|
Bovie, Valleylab, Commed
|
|
Cable length
|
5 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701389
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80100360 |
2 mm Plug protected for Resectoscope |
Erbe ACC/ICC |
5 m |
|
|

|
Cable connector instrument side
|
2 mm Plug protected for Resectoscope
|
|
Cable plug generator side
|
Erbe ACC/ICC
|
|
Cable length
|
5 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418701396
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|
|
80102131 |
5 mm Socket |
Bovie, Valleylab, Commed |
3 m |
|
|

|
Cable connector instrument side
|
5 mm Socket
|
|
Cable plug generator side
|
Bovie, Valleylab, Commed
|
|
Cable length
|
3 m
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
N/A
|
|
Regulation (EU) 2017/745
|
Class I
|
|
FDA Approval
|
Class II
|
|
GTIN 14
|
04250418738224
|
|
UMDNS No.
|
11-493
|
|
UMDNS
|
HF-Chirurgiegerät-Adapter, Kabel
|
|
GMDN No.
|
47487
|
|
GMDN
|
Medical device electrical cable, reusable
|
|
DIMDI No.
|
DE/CA39/798/02
|
|