|
89500004 |
55 mm |
|
|
|

|
Total length
|
55 mm
|
|
Dimensions
|
3x25 mm
|
|
Shaft diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728317
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500005 |
55 mm |
0.5 mm |
|
|

|
Total length
|
55 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
0.5 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728324
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500006 |
55 mm |
0.8 mm |
|
|

|
Total length
|
55 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
0.8 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728331
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500007 |
55.6 mm |
|
|
|

|
Total length
|
55.6 mm
|
|
Dimensions
|
3x24 mm
|
|
Shaft diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728348
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500008 |
43 mm |
|
|
|

|
Total length
|
43 mm
|
|
Dimensions
|
1,6x19 mm
|
|
Shaft diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728355
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500009 |
56.5 mm |
|
|
|

|
Total length
|
56.5 mm
|
|
Dimensions
|
3,3x25 mm
|
|
Shaft diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728362
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500011 |
55 mm |
0.5 mm |
|
|

|
Total length
|
55 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
0.5 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728379
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500014 |
40 mm |
5 mm |
|
|

|
Total length
|
40 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
5 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728386
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
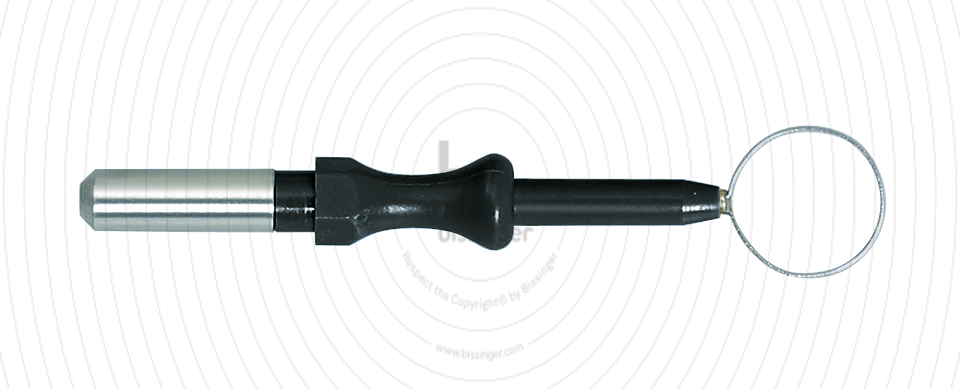
89500015 |
40 mm |
10 mm |
|
|
|
Total length
|
40 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
10 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728393
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500016 |
40.5 mm |
12 mm |
|
|

|
Total length
|
40.5 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
12 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728409
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500017 |
40.5 mm |
16 mm |
|
|

|
Total length
|
40.5 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
16 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728416
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
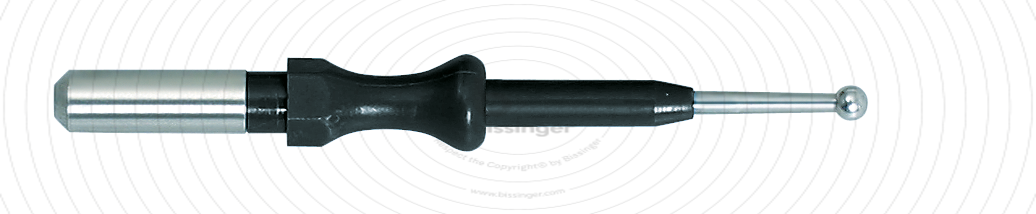
89500020 |
49.3 mm |
2 mm |
|
|
|
Total length
|
49.3 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
2 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728430
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
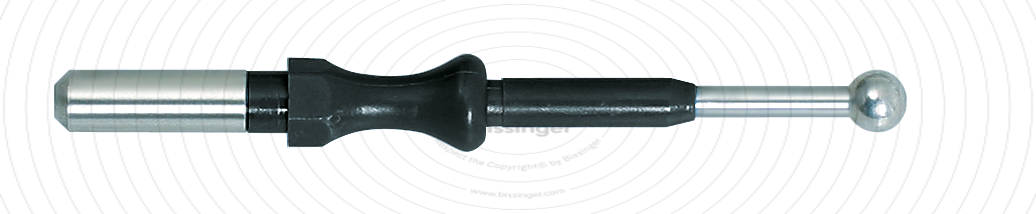
89500021 |
49.3 mm |
4 mm |
|
|
|
Total length
|
49.3 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728447
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500022 |
49.3 mm |
6 mm |
|
|

|
Total length
|
49.3 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
6 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728454
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500032 |
58 mm |
|
|
|

|
Total length
|
58 mm
|
|
Dimensions
|
2x16 mm
|
|
Shaft diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728461
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500033 |
101 mm |
|
|
|

|
Total length
|
101 mm
|
|
Dimensions
|
2x16 mm
|
|
Shaft diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728478
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500037 |
84 mm |
|
|
|

|
Total length
|
84 mm
|
|
Shaft diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728485
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500038 |
82 mm |
|
|
|

|
Total length
|
82 mm
|
|
Dimensions
|
3x24 mm
|
|
Shaft diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728492
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500122 |
53 mm |
|
|
|

|
Total length
|
53 mm
|
|
Dimensions
|
3,3x25 mm
|
|
Shaft diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728508
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500123 |
49 mm |
|
|
|

|
Total length
|
49 mm
|
|
Dimensions
|
1,5x17 mm
|
|
Shaft diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728515
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
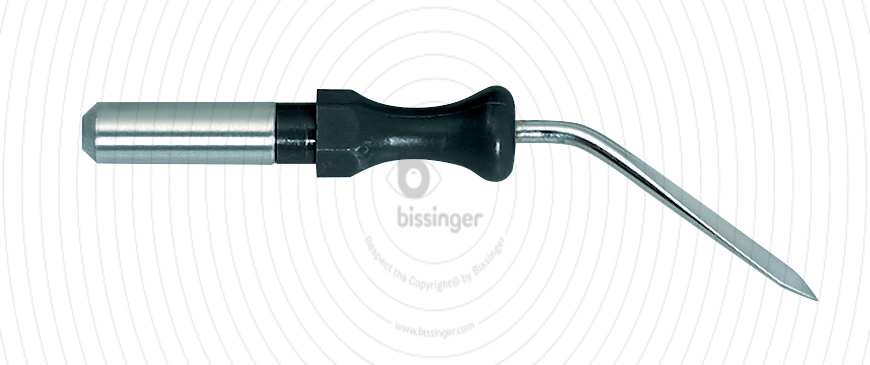
89500125 |
45 mm |
|
|
|
|
Total length
|
45 mm
|
|
Dimensions
|
1,5x17 mm
|
|
Shaft diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728522
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500126 |
54 mm |
|
|
|

|
Total length
|
54 mm
|
|
Dimensions
|
2,5x25 mm
|
|
Shaft diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728539
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500127 |
52 mm |
|
|
|

|
Total length
|
52 mm
|
|
Dimensions
|
1,6x19 mm
|
|
Shaft diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728546
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500130 |
48.5 mm |
2 mm |
|
|

|
Total length
|
48.5 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
2 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728553
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500131 |
48.5 mm |
4 mm |
|
|

|
Total length
|
48.5 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728560
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
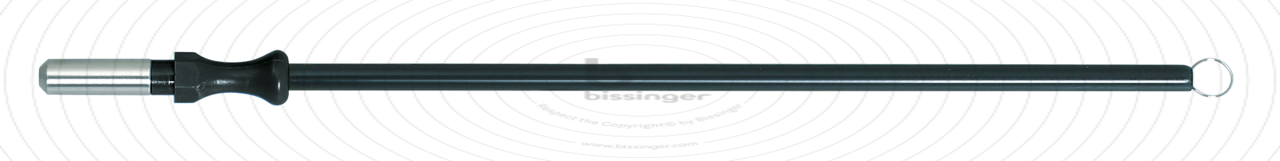
89500138 |
127 mm |
5 mm |
|
|
|
Total length
|
127 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
5 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728577
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
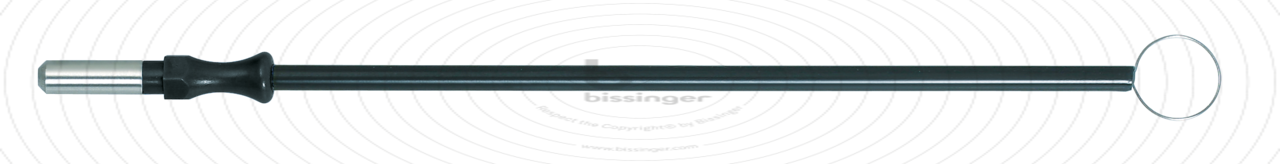
89500139 |
127 mm |
10 mm |
|
|
|
Total length
|
127 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
10 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728584
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500140 |
127 mm |
2 mm |
|
|

|
Total length
|
127 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
2 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728591
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500141 |
127 mm |
4 mm |
|
|

|
Total length
|
127 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728607
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
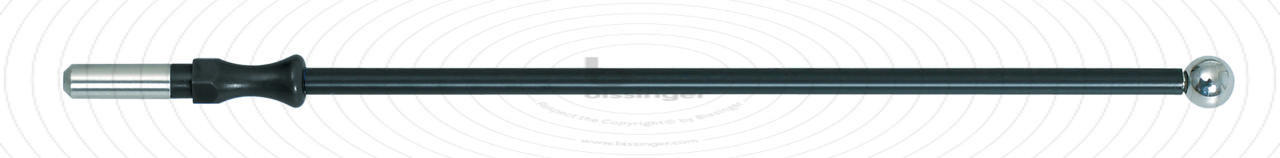
89500142 |
127 mm |
6 mm |
|
|
|
Total length
|
127 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
6 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728614
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500143 |
127 mm |
0.8 mm |
|
|

|
Total length
|
127 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
0.8 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728621
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500144 |
127 mm |
0.8 mm |
|
|

|
Total length
|
127 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
0.8 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728638
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
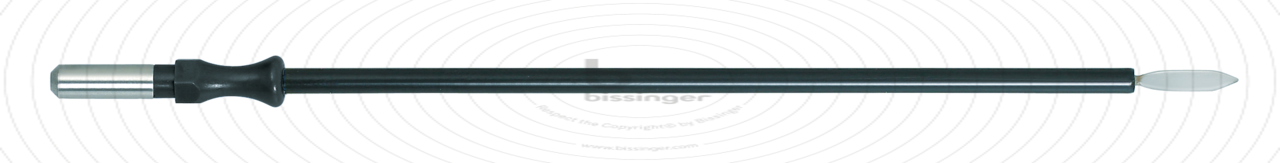
89500145 |
127 mm |
|
|
|
|
Total length
|
127 mm
|
|
Dimensions
|
2,4x10 mm
|
|
Shaft diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728645
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
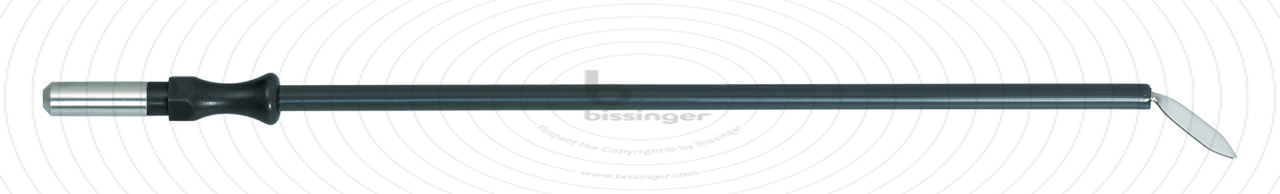
89500146 |
127 mm |
|
|
|
|
Total length
|
127 mm
|
|
Dimensions
|
2,4x10 mm
|
|
Shaft diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728652
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500147 |
127 mm |
|
|
|

|
Total length
|
127 mm
|
|
Dimensions
|
3,3x25 mm
|
|
Shaft diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728669
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
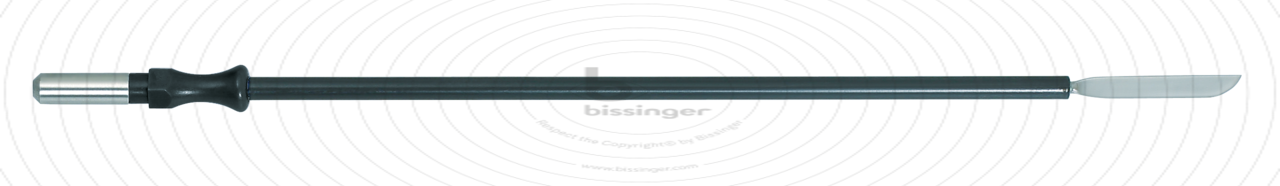
89500148 |
127 mm |
|
|
|
|
Total length
|
127 mm
|
|
Dimensions
|
3,3x20 mm
|
|
Shaft diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728676
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500149 |
127 mm |
|
|
|

|
Total length
|
127 mm
|
|
Dimensions
|
2,4x20 mm
|
|
Shaft diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728683
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500150 |
127 mm |
|
|
|

|
Total length
|
127 mm
|
|
Dimensions
|
1,6x20 mm
|
|
Shaft diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728690
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
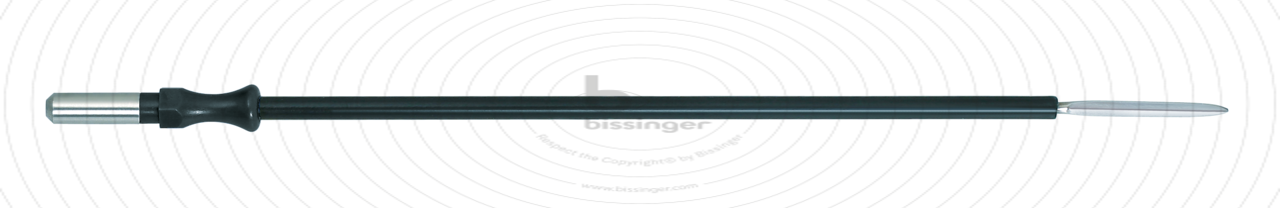
89500151 |
127 mm |
|
|
|
|
Total length
|
127 mm
|
|
Dimensions
|
2,2x20 mm
|
|
Shaft diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728706
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500170 |
142 mm |
0.6 mm |
|
|

|
Total length
|
142 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
0.6 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728713
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500171 |
142 mm |
0.6 mm |
|
|

|
Total length
|
142 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
0.6 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728720
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500172 |
150 mm |
|
|
|

|
Total length
|
150 mm
|
|
Dimensions
|
3,0x1,5 mm
|
|
Shaft diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728737
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500173 |
143 mm |
1.4 mm |
|
|

|
Total length
|
143 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
1.4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728744
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500174 |
143 mm |
3 mm |
|
|

|
Total length
|
143 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
3 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728751
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
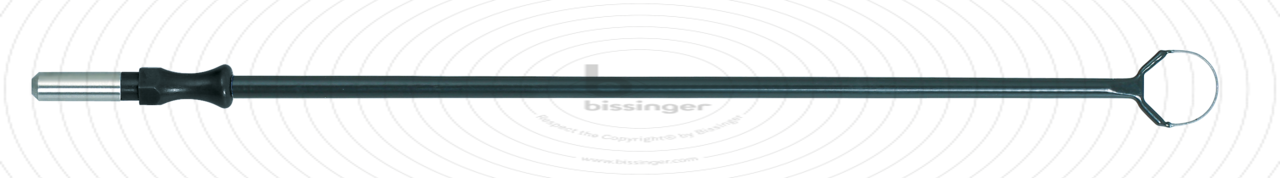
89500181 |
149.5 mm |
10 mm |
|
|
|
Total length
|
149.5 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
10 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728768
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500182 |
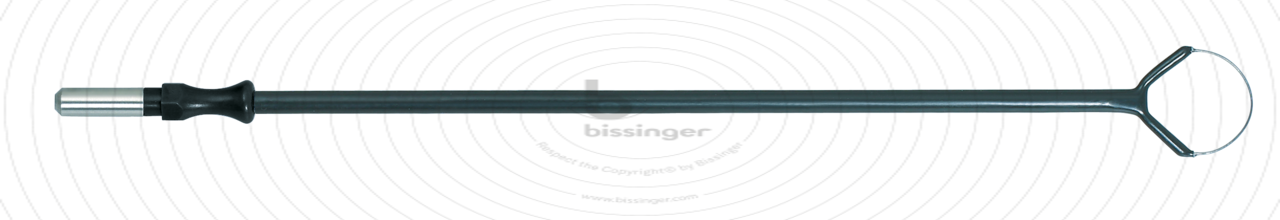
150 mm |
15 mm |
|
|
|
Total length
|
150 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
15 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728775
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500183 |
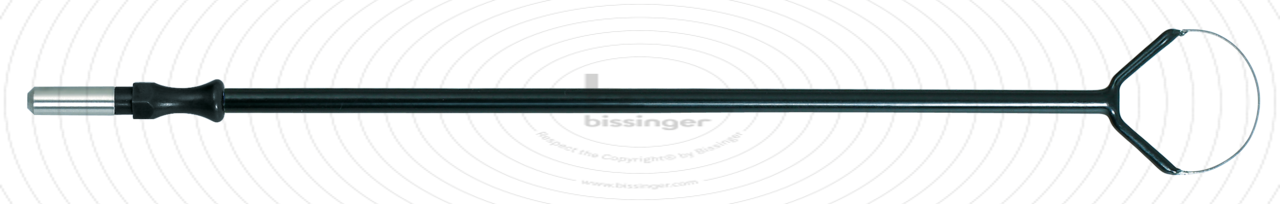
150.5 mm |
20 mm |
|
|
|
Total length
|
150.5 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
20 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728782
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500184 |
151 mm |
25 mm |
|
|

|
Total length
|
151 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
25 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728799
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500190 |
108 mm |
1.2 mm |
|
|

|
Total length
|
108 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
1.2 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728805
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89500191 |
108 mm |
2 mm |
|
|

|
Total length
|
108 mm
|
|
Shaft diameter
|
4 mm
|
|
Diameter
|
2 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728812
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|


