|
89503005 |
65 mm |
0.5 mm |
|
|

|
Total length
|
65 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
0.5 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728829
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
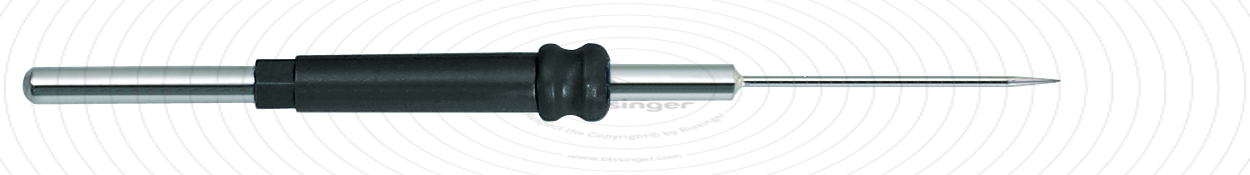
89503006 |
65 mm |
0.7 mm |
|
|
|
Total length
|
65 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
0.7 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728836
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
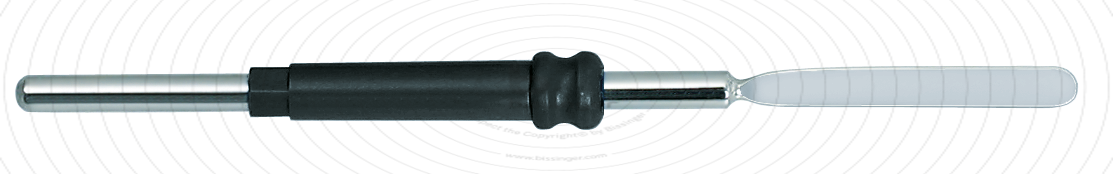
89503007 |
65 mm |
0 mm |
|
|
|
Total length
|
65 mm
|
|
Dimensions
|
2,5x20 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
0 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728843
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
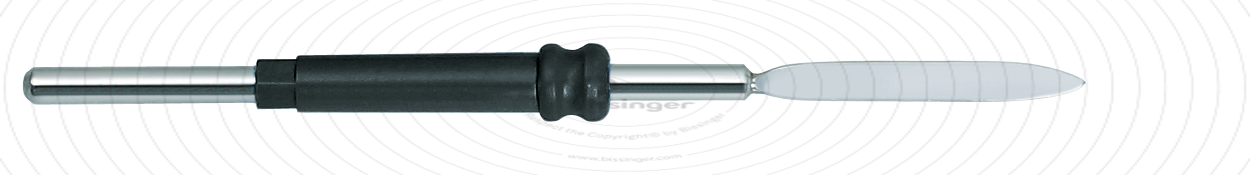
89503009 |
67 mm |
0 mm |
|
|
|
Total length
|
67 mm
|
|
Dimensions
|
2,5x20 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
0 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728850
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89503014 |
50 mm |
5 mm |
|
|

|
Total length
|
50 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
5 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728867
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89503015 |
55 mm |
10 mm |
|
|

|
Total length
|
55 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
10 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728874
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
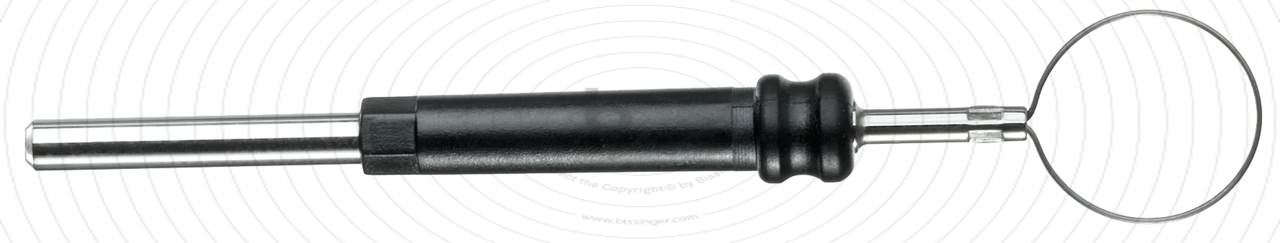
89503016 |
55 mm |
10 mm |
|
|
|
Total length
|
55 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
10 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728881
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89503017 |
59 mm |
14 mm |
|
|

|
Total length
|
59 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
14 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728898
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89503018 |
59 mm |
14 mm |
|
|

|
Total length
|
59 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
14 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728904
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
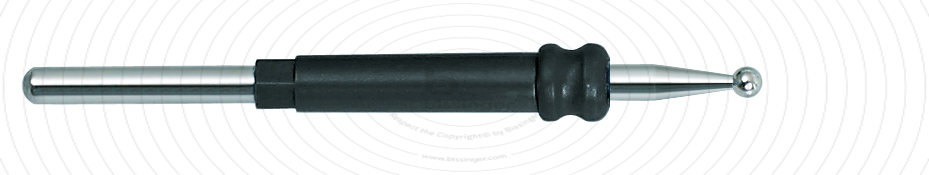
89503020 |
50 mm |
2 mm |
|
|
|
Total length
|
50 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
2 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728911
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89503021 |
50 mm |
4 mm |
|
|

|
Total length
|
50 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728928
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
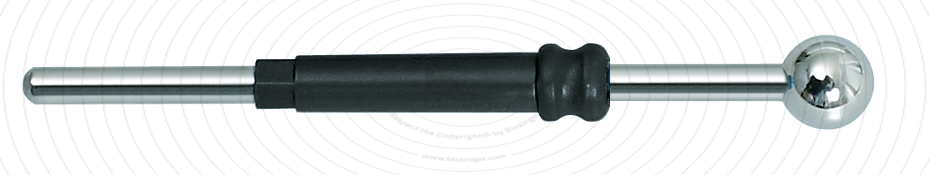
89503022 |
50 mm |
6 mm |
|
|
|
Total length
|
50 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
6 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728935
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89503130 |
49 mm |
2 mm |
|
|

|
Total length
|
49 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
2 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728942
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89503131 |
51 mm |
4 mm |
|
|

|
Total length
|
51 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418736633
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
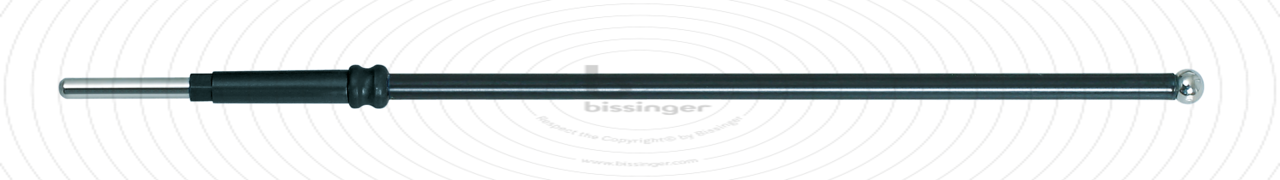
89503140 |
122 mm |
2 mm |
|
|
|
Total length
|
122 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
2 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728959
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89503141 |
124 mm |
4 mm |
|
|

|
Total length
|
124 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728966
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89503143 |
141.5 mm |
|
|
|

|
Total length
|
141.5 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728973
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89503146 |
141.5 mm |
|
|
|

|
Total length
|
141.5 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728980
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89503147 |
154 mm |
|
|
|

|
Total length
|
154 mm
|
|
Dimensions
|
2,5x20 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418728997
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89503149 |
146 mm |
|
|
|

|
Total length
|
146 mm
|
|
Dimensions
|
2,5x20 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418729000
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89503170 |
152 mm |
0.6 mm |
|
|

|
Total length
|
152 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
0.6 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418745260
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89503171 |
152 mm |
0.6 mm |
|
|

|
Total length
|
152 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
0.6 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418745277
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89503172 |
152 mm |
0.4 mm |
|
|

|
Total length
|
152 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
0.4 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418745284
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89503181 |
148.5 mm |
10 mm |
|
|

|
Total length
|
148.5 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
10 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418729017
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89503182 |
149 mm |
15 mm |
|
|

|
Total length
|
149 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
15 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418729024
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89503183 |
149.5 mm |
20 mm |
|
|

|
Total length
|
149.5 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
20 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418729031
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|
|
89503184 |
150 mm |
25 mm |
|
|

|
Total length
|
150 mm
|
|
Shaft diameter
|
2.4 mm
|
|
Diameter
|
25 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418729048
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61870
|
|
GMDN
|
Open-surgery electrodsurgical handpiece/electrode, monopolar, reusable
|
|
DIMDI No.
|
DE/CA39/798/10Ä
|
|


