| |
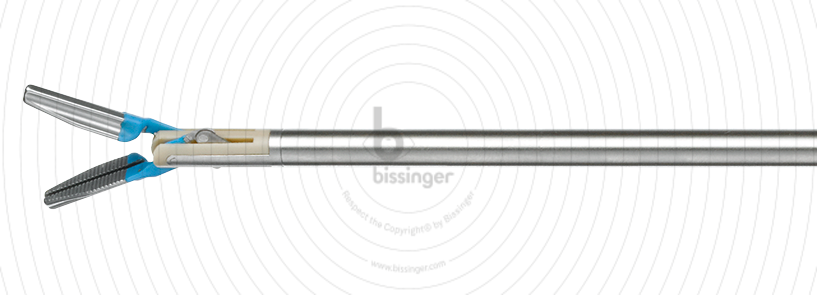
82710030 |
250 mm |
5 mm |
|
|
|
Total length
|
250 mm
|
|
Working length
|
0 mm
|
|
Diameter
|
5 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418740869
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61873
|
|
GMDN
|
Endoscopic electrosurgical electrode, bipolar, reusable
|
|
DIMDI No.
|
DE/CA39/99/037/2Ä
|
|
| |
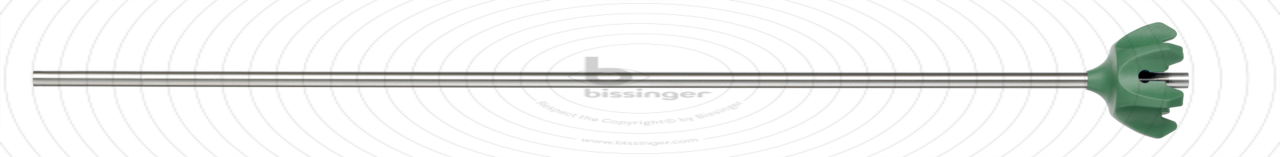
82710034 |
340 mm |
5 mm |
|
|

|
Total length
|
340 mm
|
|
Diameter
|
5 mm
|
|
Packaging unit
|
1
|
|
Reusable
|
Yes
|
|
Manual reprocessing
|
Yes, related to IFU
|
|
Directive 93/42 EEC
|
Class IIb
|
|
Regulation (EU) 2017/745
|
N/A
|
|
FDA Approval
|
N/A
|
|
GTIN 14
|
04250418737494
|
|
UMDNS No.
|
16-206
|
|
UMDNS
|
Elektrode, HF-Chirurgie, Aktivelektrode
|
|
GMDN No.
|
61873
|
|
GMDN
|
Endoscopic electrosurgical electrode, bipolar, reusable
|
|
DIMDI No.
|
DE/CA39/99/037/2Ä
|
|